The one that is extensively used as a piezoelectric material is
quartz
amorphous silica
mica
tridymite
Important Questions on Direct Current Circuits
Explain the working of Daniel cell with a neat diagram.
Consider an electrical conductor connected across a potential difference Let be a small charge moving through it in time . If is the electric current through it,
(i) the kinetic energy of the charge increases by
(ii) the electric potential energy of the charge decreases by .
(iii) the thermal energy of the conductor increases by .
Then the correct statements is/are
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
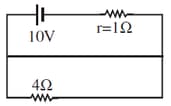
Give a brief account of an electrochemical cell. Distinguish between a primary cell and a secondary cell. Mention the factors on which the internal resistance of a cell depends.
A fuel cell converts the chemical energy of a fuel into electrical energy. In Chapter 7, you have learnt that hydrogen has a very high calorific value, but cannot be used the way we use other fuels because it forms an explosive mixture with air. A fuel cell is a safe way of utilising the chemical energy of hydrogen. What is great about this idea is that hydrogen can be produced by the electrolysis of water, and water is not in short supply the way fossil fuels are.
In a hydrogen fuel cell, hydrogen splits into ions and electrons at the anode. The hydrogen ions pass through a selective membrane to the cathode. The electrons, on the other hand, travel along the external circuit (outside the cell) from the cathode to the anode, giving rise to a current that we can draw. At the anode, the hydrogen ions combine with electrons (that have travelled through the external circuit) and oxygen to form water. This is the other great thing about a hydrogen fuel cell—the only waste produced is water, which is not a pollutant. So, why have we not started using fuel cells on a large scale? That is because they are not yet efficient and inexpensive enough for common use.
Explain briefly how a hydrogen fuel cell works.
Reason: There is no field inside the cell, when the cell is in open circuit.

