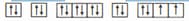MEDIUM
11th CBSE
IMPORTANT
Earn 100
The orbitals which have same number of nodes are
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
The diagram illustrates a possible electronic configuration of which of the following species
EASY
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT