EASY
Earn 100
The and hydrogen differ in respect of which of the following?
50% studentsanswered this correctly
Important Questions on Hydrogen and its Compounds
EASY
EASY
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Hydrogen is the most abundant element in the Universe, but it is not the most abundant gas in the troposphere.
Reason R: Hydrogen is the lightest element.
In the light of the above statements, choose the correct answer from the options given below:
MEDIUM
EASY
EASY
Oxygen (61.4%); Carbon (22.9%), Hydrogen (10.0%); and Nitrogen (2.6%). The weight which a 75kg person would gain if all atoms are replaced by atoms is:
EASY
EASY
EASY
EASY
Which of the following statements about hydrogen is incorrect?
EASY
1) It exists as a diatomic molecule.
2) It has one electron in the outermost shell.
3) It can lose an electron to form a cation which can freely exist.
4) It cannot form ionic compounds.
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
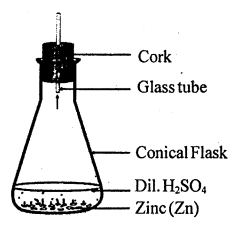
MEDIUM
Out of the oxides given below which of them cannot be reduced by ?
EASY
EASY
EASY
