MEDIUM
12th ICSE
IMPORTANT
Earn 100
The osmotic pressure of equimolal solutions of glucose, sodium chloride and barium chloride will be in the order
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
Consider the following figure and choose the correct option:
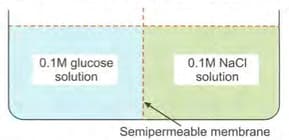
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
