The values of some substances are given in the table. Analyse the table and answer the question.
Substance
value
Vinegar
Lime water
Milk
Water
Toothpaste
Blood
Is blood acidic or basic in nature?

Important Questions on Acids, Bases, Salts
The values of some substances are given in the table. Analyse the table and answer the question.
| Substance | value |
| Vinegar | |
| Lime water | |
| Milk | |
| Water | |
| Toothpaste | |
| Blood |
The value of pure milk is . Does the value increase or decrease when milk changes to curd. Justify your answer
The values of some substances are given in the table. Analyse the table and answer the question.
| Substance | value |
| Vinegar | |
| Lime water | |
| Milk | |
| Water | |
| Toothpaste | |
| Blood |
Among the substances given in the above table
(i) Which one is strongly basic?
(ii) Which one has weak acidic nature?
Organic acids are present in a number of substances we use in our daily life.
(e.g. Tomato, orange, apple, grapes, curd, etc.)
Identify the organic acids in each of them and tabulate.
Complete the equations of the ionisation of phosphoric acid.
(Dihydrogen phosphate ion)
_____ (Hydrogen phosphate ion)
_____ (phosphate ion)
Write the chemical name of the following salts.
Solution of sodium carbonate, potassium chloride, and ammonium sulphate are taken in separate beakers.
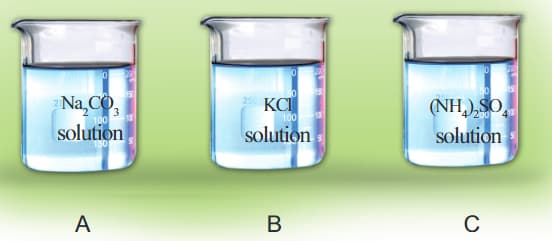
Recall the colour change of litmus paper in each solution and tabulate.
| Salt | Colour of the litmus paper | Nature of the substance |
Solution of sodium carbonate, potassium chloride, and ammonium sulphate are taken in separate beakers.
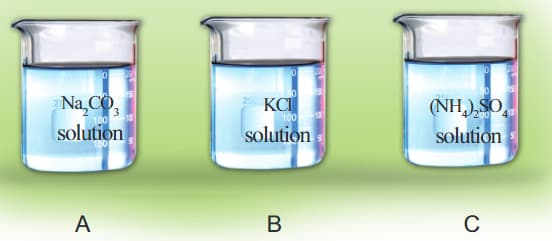
Name the acid and alkali that react to form each salt given above?
