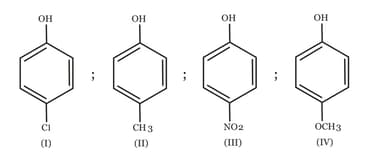
The value of four halo phenols is given below:
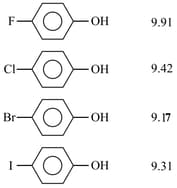
Consider the following statements regarding value:
(1) and atoms have both and effects
(2) In case of -chloro, -bromo, and -iodophenoxide ions, and acts as accept (- effect)
(3) Due to acceptor nature of halogen atom, the negative charge on oxygen of phenoxide ion gets delocalised and hence effect predominates.
(4) The accepting ability order is as . Hence -iodophenol is most acidic.
Of the these the correct statements are :

Important Questions on Alcohols, Phenols and Ethers
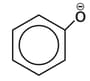 ion ?
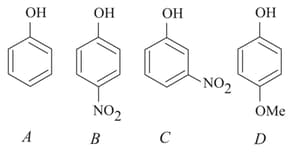
ion ?The increasing order of the values of the following compounds is:
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
Match the following values.
| Acid | |||
| (a) | Phenol | (i) | |
| (b) | -Nitrophenol | (ii) | |
| (c) | Ethanol | (iii) | |
| (d) | Picric acid | (iv) |
the compound is:
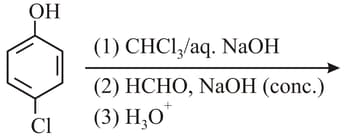
What is the product "C" after following reactions -
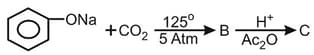
The major product of the following reaction is:
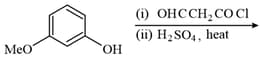
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide

