EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
Earn 100
The packing efficiency of the face centred cubic , body centred cubic and simple/primitive cubic lattices follows the order
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solid State
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
A unit cell of calcium fluoride has four calcium ions. The number of fluoride ions in the unit is
MEDIUM
KVPY Aptitude Test - Stream SX
IMPORTANT
Copper (atomic mass ) crystallizes in lattice and has density . The radius of copper atom is closest to
MEDIUM
KVPY Aptitude Test - Stream SX
IMPORTANT
A mineral consists of a cubic close-packed structure formed by ions, where half the octahedral voids are occupied by and one-eighth of the tetrahedral voids are occupied by . The chemical formula of the
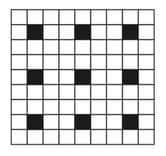
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
A two-dimensional solid pattern formed by two different atoms and is shown below. The black and white squares represent atoms and , respectively. The simplest formula for the compound based on the unit cell from the pattern is