MEDIUM
NEET
IMPORTANT
Earn 100
The percentage of character in the orbitals forming bonds in is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
NEET
IMPORTANT
Assuming the Hund's rule violated, the bond order and magnetic nature of the diatomic molecule of is:
MEDIUM
NEET
IMPORTANT
Assuming 2s-2p mixing is not operative, the paramagnetic species among the following is
EASY
NEET
IMPORTANT
Arrange the following in the increasing order of deviation from the normal tetrahedral angle:
HARD
NEET
IMPORTANT
Which compound among the following has least ionic character?
HARD
NEET
IMPORTANT
The dipole moment of 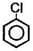 is Debye. The dipole moment of
is Debye. The dipole moment of 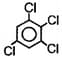 will be -
will be -
MEDIUM
NEET
IMPORTANT
Which of the following compound has Zero dipole moment -
EASY
NEET
IMPORTANT
Which of the following compounds posses highest dipole moment.
EASY
NEET
IMPORTANT
How many and bonds are present in respectively?
