EASY
Earn 100
The photoelectric effect represent that ...........
(a)Light has a particle nature
(b)Proton has a wave nature
(c)Electron has a wave nature
(d)None of these
66.67% studentsanswered this correctly
Important Questions on Dual Nature of Matter and Radiation
EASY
EASY
EASY
EASY
The photoelectric threshold for a certain metal surface is . If the metal surface is irradiated by a wavelength of , the kinetic energy of the emitted photoelectrons is
EASY
MEDIUM
EASY
Given (in )
EASY
EASY
EASY
( = Planck's constant, = speed of light)
EASY
(Assume mass of electron and Charge of electron )
MEDIUM
EASY
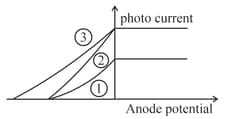
The following graph represents the variation of photo current with anode potential for a metal surface. Here and represents intensities and represents frequency for curves and respectively, then
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM

