MEDIUM
Earn 100
The picture shows an incomplete periodic table.
Arrow 1 and arrow 2 indicate increase or decrease in chemical properties.
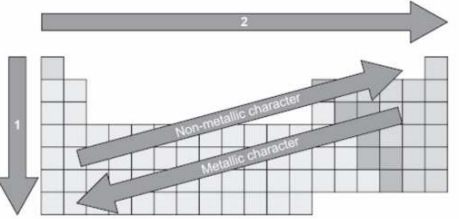

(a)
Arrow 1-Decrease in atomic mass
Arrow 2-Increase in atomic radius
(b)
Arrow 1-Increase in atomic radius
Arrow 2-Increase in electronegativity
(c)
Arrow 1-Increase in electronegativity
Arrow 2-Decrease in atomic radius
(d)
Arrow 1-Decrease in atomic radius
Arrow 2-Decrease in atomic mass
50% studentsanswered this correctly
Important Questions on Periodic Classification of Elements
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
EASY
MEDIUM
HARD
EASY
EASY
EASY
Moseley classified elements according to their _____. (atomic number/atomic mass)
HARD
EASY
MEDIUM
EASY
MEDIUM
EASY
HARD
MEDIUM

