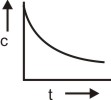
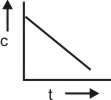
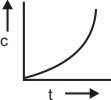
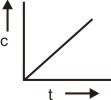
The plot between concentration versus time for a zero-order reaction is represented by______.

Important Questions on Chemical Kinetics
The relationship between rate constant and half life period of zero order reaction is given by_____.
The time for half life period of a certain reaction , is . The initial concentration of the reactant is . How much time will it take for its concentration to come from , if it is a zero order reaction______.
Units of rate constant of first and zero order reaction in terms of molarity unit are, respectively...?
Certain bimolecular reactions, which follow the first-order kinetics are called _____.
Which among the following reactions is an example of pseudo first-order reaction?
Number of reactant molecules participating in a chemical reaction is called _____.
For the reaction , if the rate law expression is, rate = . The molecularity and order of the reaction are, respectively ______.
A certain reaction occurs in two steps as:
In the reaction, _____
