The polymerization of ethylene to linear polyethylene is represented by the reaction,
, where has a large integral value. Given that the average enthalpies of bond dissociation for and at are and , respectively, calculate the enthalpy of polymerization per mole of ethylene at .

Important Questions on Miscellaneous Problems for Revision
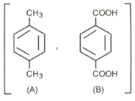
A hydrocarbon , Vapour density was subjected to vigorous oxidation to give a dibasic acid . of required of for the complete neutralization. The nitration of gave a single mononitro derivative. When was heated strongly with soda lime, it gave benzene. Identify and .
A chloro compound showed the following properties:
Decolourised bromine in
Absorbed hydrogen catalytically
Gave a precipitate with ammonium cuprous chloride
When vapourised, of gave of vapour at .
Identify .
How many grams of must be dissolved in of water at to make the solution saturated?
(Molar mass of )
Answer after rounding-off the answer up to four digits after decimal.
A solution which is in and in , is treated with solid . Which compound, or , will precipitate first? What is the concentration of the anion of the least soluble compound, when the more soluble one starts precipitating?
Given:
