EASY
Earn 100
The positron is antiparticle of
(a)Electron
(b)Proton
(c)Neutron
(d)Antiproton
50% studentsanswered this correctly
Important Questions on Atomic Structure and Particle Physics
EASY
MEDIUM
HARD
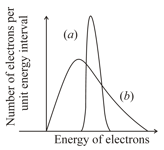
Originally the radioactive beta decay was thought as a decay of a nucleus with the emission of electrons only (Case I) . However, in addition to the electron, another (nearly) massless and electrically neutral particle is also emitted (Case II). Based on the figure below, which of the following is correct?
EASY
EASY
HARD
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
The probability of survival of a radioactive nucleus for one mean life is:
EASY
In - ray emission from a nucleus
EASY
EASY
A sample of which is - emitter with days is observed by a student to have 2000 disintegrations (2000 Bq). What is the mass of the sample ?

