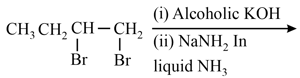EASY
Earn 100
The presence of ion increases the solubility of alkenes due to the formation of
(a) bonding
(b) bonding
(c) bonding
(d) bonding
61.9% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
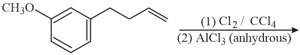
The major product of the following reaction is:
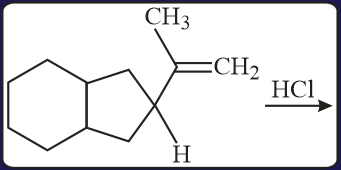
HARD
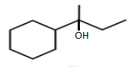 ?
?MEDIUM
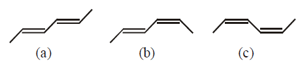
HARD
HARD
The major product in the following conversion is
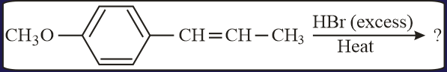
MEDIUM
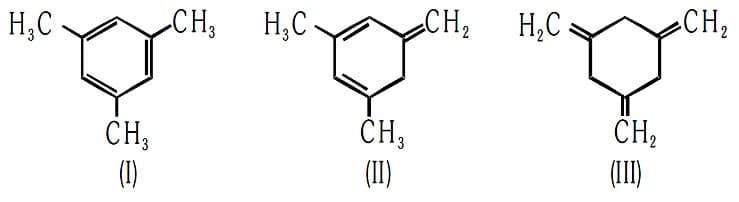
The enthalpy of hydrogenation of these compounds will be in the order as:
EASY
MEDIUM
MEDIUM
HARD
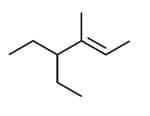
What is the major product expected from the following reaction?
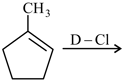
Where D is an isotope of hydrogen.
EASY
HARD
MEDIUM
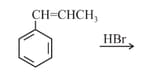
EASY