MEDIUM
JEE Main
IMPORTANT
Earn 100
The pressure and density of diatomic gas changes suddenly to and respectively during an adiabatic process. The temperature of the gas increases and becomes____times of its initial temperature.
(Given )
100% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
One mole of a monoatomic gas is mixed with three moles of a diatomic gas. The molecular specific heat of mixture at constant volume is ; then the value of will be _____ . (Assume that the given diatomic gas has no vibrational mode.)
MEDIUM
JEE Main
IMPORTANT
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in the figure. Its volume is then reduced to the original volume from to by an isobaric process. The total work done by the gas from to to will be
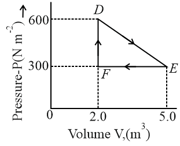
MEDIUM
JEE Main
IMPORTANT
of a liquid is converted to vapour at pressure. If of the heat supplied is used for increasing the volume by during this phase change, then the increase in internal energy in the process will be :
EASY
JEE Main
IMPORTANT
Let be the ratio of molar specific heat at constant pressure and molar specific heat at constant volume of a monoatomic gas and be the similar ratio of diatomic gas. Considering the diatomic gas molecule as a rigid rotator, the ratio is:
MEDIUM
JEE Main
IMPORTANT
In an Isothermal change, the change in pressure and volume of a gas can be represented for three different temperature; as:
MEDIUM
JEE Main
IMPORTANT
A Carnot engine with efficiency takes heat from a source at . In order to increase the efficiency to , keeping the temperature of sink same, the new temperature of the source will be:
EASY
JEE Main
IMPORTANT
According to law of equipartition of energy the molar specific heat of a diatomic gas at constant volume where the molecule has one additional vibrational mode is :-
MEDIUM
JEE Main
IMPORTANT
Match List I with List II :
| List I | List II | ||
| A | Isothermal Process | I | Work done by the gas decreases internal energy |
| B | Adiabatic Process | II | No change in internal energy |
| C | Isochoric Process | III | The heat absorbed goes partly to increase internal energy and partly to do work |
| D | Isobaric Process | IV | No work is done on or by the gas |
Choose the correct answer from the options given below :
