HARD
11th West Bengal Board
IMPORTANT
Earn 100
The pressure and density of a diatomic gas changes adiabatically from to . If , then be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
11th West Bengal Board
IMPORTANT
MEDIUM
11th West Bengal Board
IMPORTANT
In figures we see diagrams for cyclic processes for a gas. In which of these processes is heat absorbed by the gas ?
MEDIUM
11th West Bengal Board
IMPORTANT
EASY
11th West Bengal Board
IMPORTANT
HARD
11th West Bengal Board
IMPORTANT
HARD
11th West Bengal Board
IMPORTANT
Two moles of helium gas has passed through a cyclic process shown in the diagram in figure (Fig. ). If the gas is ideal, work done on the gas for the process to is for the gas is
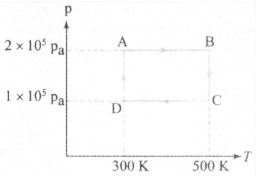
HARD
11th West Bengal Board
IMPORTANT
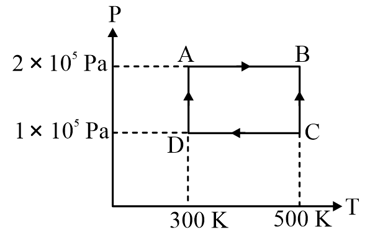
In the above cycle, work done on the gas in the process to is
HARD
11th West Bengal Board
IMPORTANT
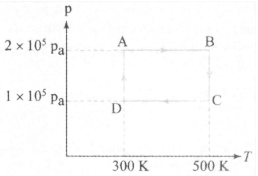
In the above cycle, work done on the gas in the whole cycle is
