EASY
Earn 100
The pressure and volume of an ideal gas are related as (Constant). The work done when the gas is taken from state to state is :
(a)
(b)
(c)
(d)
52.73% studentsanswered this correctly
Important Questions on Thermodynamics
HARD
HARD
The equation of state of moles of a non-ideal gas can be approximated by the equation where, and, are constant characteristics of the gas. Which of the following can represent the equation of a quasi-static adiabatic for this gas (assume that, is the molar heat capacity at constant volume is independent of temperature)?
MEDIUM
Two moles of an ideal monoatomic gas occupies a volume at . The gas expands adiabatically to a volume . Calculate the final temperature of the gas and change in its internal energy.
EASY
HARD
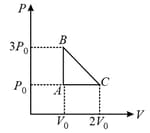
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 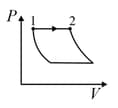 |
| (II) | (ii) Isochoric | (Q) 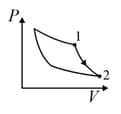 |
| (III) | (iii) Isobaric | (R) 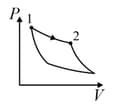 |
| (IV) | (iv) Adiabatic | (S) 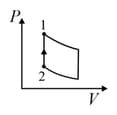 |
EASY
MEDIUM
HARD
MEDIUM
EASY
HARD
MEDIUM
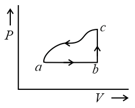
EASY
HARD
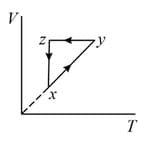
The - diagram that best describes this cycle is: (Diagrams are schematic and not to scale)
MEDIUM
Match the thermodynamics processes taking place in a system with the correct conditions. In the table : is the heat supplied, is the work done and is change in internal energy of the system.
| Process | Condition | ||
| (I) | Adiabatic | (A) | |
| (II) | Isothermal | (B) | |
| (III) | Isochoric | (C) | |
| (IV) |
Isobaric |
(D) |
MEDIUM
EASY
EASY
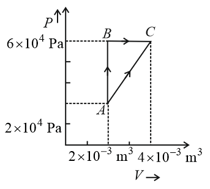
In process AB, of heat is added to the system and in process BC, of heat is added to the system. The heat absorbed by the system in the process AC will be:
MEDIUM
The corresponding P - V diagram for the process is (all figures are schematic and not drawn to scale) :

