MEDIUM
Earn 100
The pressure exerted by 12 gm of an ideal gas at in a vessel of volume v litre is one atm. When the temperature is increased by at the same volume the pressure increased by 10%. Calculate the temperature t and volume v? (Mol. Wt. = 120)
(a)0.82
(b)2.88
(c)3.78
(d)1.80
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
EASY
MEDIUM
MEDIUM
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
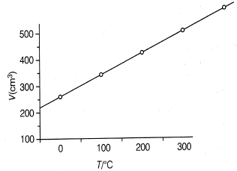
The volume of the gas at is larger than that at by a factor of
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
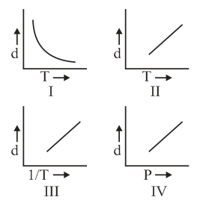
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
MEDIUM
HARD
MEDIUM
[Gas constant, ]
MEDIUM
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

