HARD
JEE Advanced
IMPORTANT
Earn 100
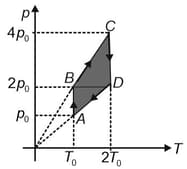
The pressure versus temperature graph of of an ideal gas is shown in the figure. Plot the corresponding,
density versus volume graph
pressure versus volume graph and
density versus pressure graph.
density versus volume graph
pressure versus volume graph and
density versus pressure graph.


Important Questions on Laws of Thermodynamics
HARD
JEE Advanced
IMPORTANT
Three moles of an ideal gas being initially at a temperature were isothermally expanded times its initial volume and then isochorically heated so that the pressure in the final state becomes equal to that in the initial state. The total heat supplied in the process is . Find of the gas.
EASY
JEE Advanced
IMPORTANT
ice at is converted into steam at . Find the total heat required..
EASY
JEE Advanced
IMPORTANT
Three liquids , and of specific heats , and are at temperatures , and , respectively. Find temperature in equilibrium if they are mixed together. Their masses are equal.
