The process of boiling a liquid above its boiling point is called .
Important Questions on Calorimetry and Change of State
Answer the following questions based on the P–T phase diagram of
is heated to a temperature and compressed isothermally. What changes in its properties do you expect to observe?
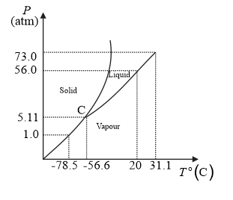
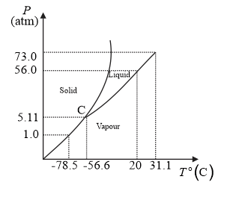
The following questions based on the phase diagram of carbon dioxide:
Is solid, liquid or gas at (a) under , (b) under , (c) under ?
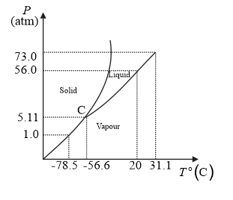
Answer the following questions based on the P–T phase diagram of
What happens when at pressure is cooled from room temperature at constant pressure?
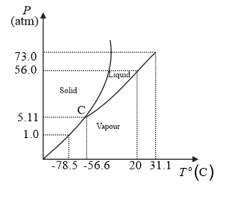
Answer the following questions based on the P–T phase diagram of :
at 1 atm pressure and temperature is compressed isothermally. Does it go through a liquid phase?
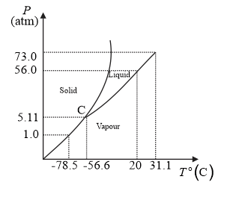
Answer the following questions based on the P–T phase diagram of
Describe qualitatively the changes in a given mass of solid at pressure and temperature as it is heated up to room temperature at constant pressure.
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
Assertion (A): The boiling point of water decreases as the altitude increases.
Reason (R): The atmospheric pressure increases with altitude.
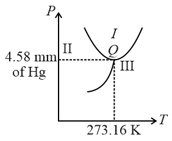
The triple-point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and the boiling point of water as standard fixed points (as was originally done in the Celsius scale) ?
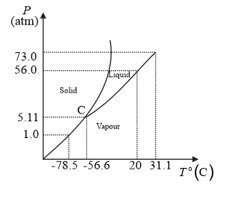
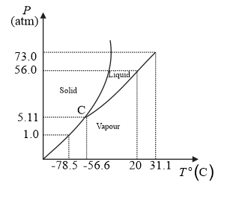
The following questions based on the phase diagram of carbon dioxide:
What are the critical temperature and pressure for ? What is their significance?
The following questions based on the phase diagram of carbon dioxide:
At what temperature and pressure can the solid, liquid and vapour phases of co-exist in equilibrium?
The pair of boiling point and compound are given as,
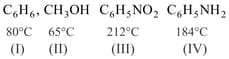
Which will show lowest vapour pressure at room temperature?