EASY
Earn 100
The product formed by the reaction of Copper sulphate with sodium hydroxide is:
(a)Copper chloride
(b)Copper hydroxide
(c)Copper sulphate
(d)None of the above
50% studentsanswered this correctly
Important Questions on Inorganic Qualitative Analysis
MEDIUM
MEDIUM
For the following Assertion and Reason, the correct option is
Assertion (A) : When (II) and sulphide ions are mixed, they react together extremely quickly to give a solid.
Reason (R) : The equilibrium constant of is high because the solubility product is low.
EASY
EASY
| List - I (Metal ion) | List - II (Group in Qualitative analysis) | ||
| (a) | (i) | Group - III | |
| (b) | (ii) | Group - IIA | |
| (c) | (iii) | Group - IV | |
| (d) | (iv) | Group - IIB |
MEDIUM
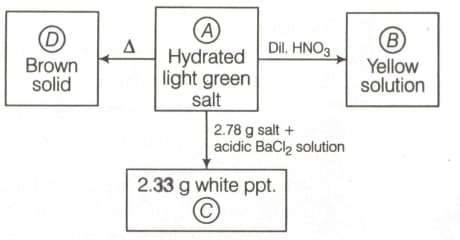
HARD
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
EASY
MEDIUM