HARD
Earn 100
The product obtained by heating zinc blende in air is produces [A], [A] is dissolved in dil. H2SO4 produces [B]. A mixture of [B] and BaS is used as white pigment. Choose the incorrect statement among following statements.
(a)[B] is known as white vitriol when it contain 7 moles of water of crystallisation
(b)[B] can also be prepared by reaction of zinc carbonate with dil sulphuric acid
(c)A mixture of [B] and barium sulphate is known as lithophone
(d)[A] is zinc oxide and amphoteric in nature
50% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
MEDIUM
MEDIUM
Consider the following statements relating to Sea Salinity:
1. The ocean salinity depends on evaporation and precipitation.
2. Any change in the temperature or density influences the salinity.
3. Major source of sea salinity of terrestrial discharge by rivers.
Which of the statements given above are correct? Select the correct answer using the codes below:
MEDIUM
In the given reaction cycle
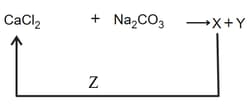
and respectively are
MEDIUM
EASY
Write a balanced chemical equation the following reaction:
Action of dilute sulphuric acid on zinc sulphide.
MEDIUM
EASY
EASY
EASY
EASY
Which one of the following chemical reactions is not feasible?
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
Write a balanced equation for the preparation of the following salt: Copper sulphate from Copper carbonate.
EASY

