EASY
NEET
IMPORTANT
Earn 100
The radius of (Atomic number of ) is Angstrom. Which one of the following given values will be closest to the radius of (Atomic number of )?
(a) Angstrom
(b) Angstrom
(c) Angstrom
(d) Angstrom
50% studentsanswered this correctly

Important Questions on The d- and f-Block Elements
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
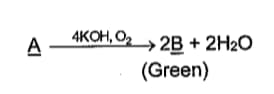

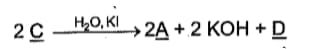
In the above sequence of reactions, and , respectively, are:
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
