MEDIUM
Earn 100
The rate constant for a chemical reaction has units ,order of the reaction will be
(a)0
(b)1
(c)2
(d)3
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
EASY
MEDIUM
(i)
(ii)
(iii)
The overall order of the reaction will be
HARD
For an elementary chemical reaction, , the expression for is:
HARD
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
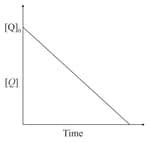
EASY
MEDIUM
HARD
(Assume that all these gases behave as ideal gases)
EASY
EASY
EASY
HARD
EASY
HARD
EASY
If the concentration of is increased from , keeping the value of at , the rate constant will be:
MEDIUM
HARD
MEDIUM
Which one of the following statements is correct?
MEDIUM

