The rate constant of a reaction is at and its activation energy is . What is the value of the rate constant at ?

Important Questions on Sample Question Paper 5
Nitrogen pentoxide decomposes according to the following equation, . This first order reaction was allowed to proceed at and the data given below:
| Time | |
Calculate the rate constant.
Nitrogen pentoxide decomposes according to the following equation, . This first order reaction was allowed to proceed at and the data given below:
| Time | |
What will be the concentration of after ?
Nitrogen pentoxide decomposes according to the following equation, . This first order reaction was allowed to proceed at and the data given below:
| Time | |
Calculate the initial rate of reaction.
For a chemical reaction, variation in concentration versus time plot is given below :
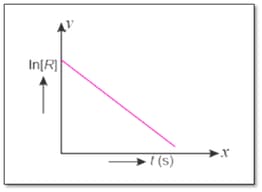
What is the order of reaction?
For a chemical reaction, variation in concentration versus time plot is given below :
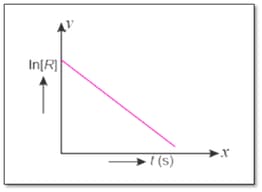
What are the units of rate constant for the reaction?
For a chemical reaction, variation in concentration versus time plot is given below :
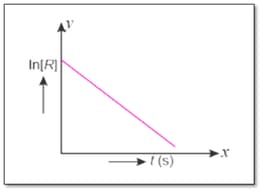
If the initial concentration of the reactant is half of the original concentration, how will change?
For a chemical reaction, variation in concentration versus time plot is given below :
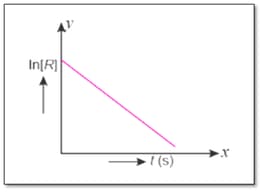
Draw the plot of log versus time .
