MEDIUM
Earn 100
The rate law for the reaction is given by
Rate . The rate of the reaction will be
(a)Decreased on increasing the temperature of the reaction
(b)Halved on reducing the concentration of alkyl halide to one half
(c)Doubled on doubling the concentration of sodium hydroxide
(d)Unaffected by increasing the temperature of the reaction
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
For an elementary chemical reaction, , the expression for is:
MEDIUM
In the following reaction;
‘A’ and ‘B’ respectively can be:
MEDIUM
EASY
Consider the following reactions
The order of the above reactions are respectively. The following graph is obtained when log[rate] vs.log[conc.] are plotted:
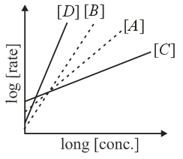
Among the following, the correct sequence for the order of the reactions is :
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
HARD
The results given in the below table were obtained during kinetic studies of the following reaction:
| Experiment | Initial rate/ | ||
| I | |||
| II | |||
| III | |||
| IV | X | ||
| V | Y |
X and Y in the given table are respectively :
EASY
EASY
EASY
If the concentration of is increased from , keeping the value of at , the rate constant will be:
HARD
...(i)
...(ii)
The closest rate constant for the overall reaction
is:
EASY
Which of the following expression is correct for the rate of reaction given below ?
MEDIUM
MEDIUM
MEDIUM
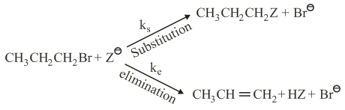
where,
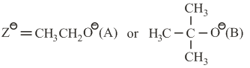
and , are respectively, the rate constants for substitution and elimination, and , the correct option is ________
MEDIUM
MEDIUM
EASY
EASY
The initial concentration of is and it is after 30 minutes. The rate of formation of is:
MEDIUM
(i)
(ii)
(iii)
The overall order of the reaction will be
EASY

