The rate of disappearance of the substrate is proportional to the concentration of substrate up to a certain substrate concentration.
Important Questions on Enzymes
After a cut, the body responds by forming a blood clot, platelets release thromboplastin and an enzyme-controlled reaction begins.
Why does this cause the rate of blood clotting to increase?
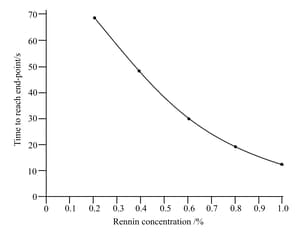
Why is it better to calculate the initial rate of reaction from a curve such as the one in. In given figure how much oxygen is given off in 30 seconds?
A student investigated the effect of several different catalysts on the rate of decomposition of hydrogen peroxide to water and oxygen. The speed of the reaction was judged by how 'fizzy' or frothy the contents of the tube became when the catalyst was added (oxygen is a product of the reaction and forms bubbles). The student used iron filings and manganese dioxide as inorganic catalysts. They also used a commercial preparation of the enzyme catalase and pieces of liver and pieces of potato tuber, both of which contain catalase. Catalase catalyses the decomposition of hydrogen peroxide.
Results showed: • catalase, liver and potato were much more efficient than the inorganic catalysts • pure catalase was more efficient than the liver and potato • liver was more efficient than potato • ground-up liver was more efficient than pieces of liver. Try to explain the student's results.
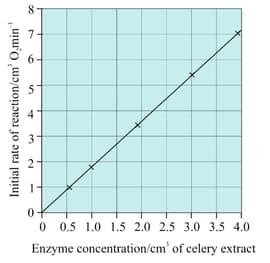
Use your knowledge and understanding of enzyme activity to explain the results shown in the given graph.