EASY
Earn 100
The rate of oxidation of oxalic acid by acidified potassium permanganate is shown in the given graph.
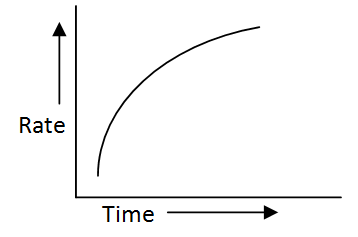
The type of catalyst shown by such reaction is
(a)heterogeneous catalysis
(b)induced catalysis
(c)auto-catalysis
(d)negative catalysis
50% studentsanswered this correctly
Important Questions on Surface Chemistry
HARD
| List - Reaction | List - Catalyst | ||
The correct answer is:
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
| Column I | Column II | ||
|---|---|---|---|
| Catalyst | Product | ||
| A. | P. | Polyethylene | |
| B. | Q. | Ethanal | |
| C. | R. | ||
| D. | Iron Oxide | S. |
MEDIUM
MEDIUM
Match List with List
| List (process) | List (catalyst) | ||
| (a) | Deacon's process | (i) | |
| (b) | Contact process | (ii) | |
| (c) | Cracking of hydrocarbons | (iii) | |
| (d) | Hydrogenation of vegetable oils | (iv) |
Choose the most appropriate answer from the options given below-
HARD
HARD
HARD
EASY
MEDIUM

