The rate of the reaction between magnesium and dilute hydrochloric acid can be measured using this apparatus:
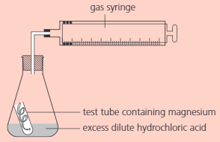
What is the purpose of the test-tube?


Important Questions on The Speed of a Reaction
The rate of the reaction between magnesium and dilute hydrochloric acid can be measured using this apparatus:
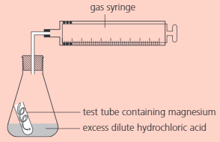
What is the purpose of the gas syringe?
The rate of the reaction between magnesium and dilute hydrochloric acid can be measured using this apparatus:
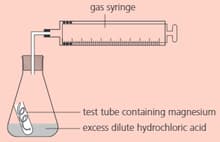
How would you get the reaction to start?
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
What does an excess of acid mean?
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
Plot a graph of the results.
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
What is the rate of reaction (in of hydrogen per minute) during the first minute?
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
What is the rate of reaction (in of hydrogen per minute) during the second minute?
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
What is the rate of reaction (in of hydrogen per minute) during the third minute?
Some magnesium and an excess of dilute hydrochloric acid were reacted together. The volume of hydrogen produced was recorded every minute, as shown in the table:
| Time / min | ||||||||
| Volume of hydrogen / |
Why does the rate change during the reaction?
