HARD
JEE Main
IMPORTANT
Earn 100
The ratio of mass percent of and of an organic compound is . If one molecule of the above compound contains half as much oxygen as required to burn one molecule of compound completely to and , what will be the empirical formula of the compound ?
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
Excess of calcium orthophosphate is reacted with magnesium to form calcium phosphide with magnesium oxide. Calcium phosphide on reacting with an excess of water liberate phosphine gas () along with calcium hydroxide. The evolved phosphine was burnt in the air (excess of oxygen ) to yield phosphorus pentoxide along with water. How many grams of magnesium metaphosphate would be obtained, if of magnesium were used for reducing calcium phosphate:
( Atomic wt. of )
Magnesium metaphosphate
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
Which of the following atoms/molecules are placed at the correct position?
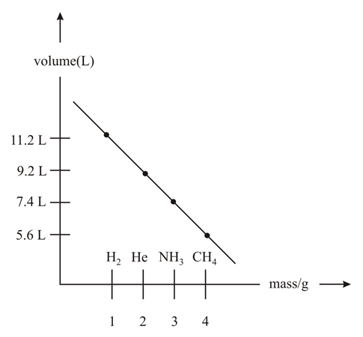
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
