EASY
JEE Main
IMPORTANT
Earn 100
The ratio of radii of the first three Bohr's orbits is
(a)
(b)
(c)
(d)
69.7% studentsanswered this correctly

Important Questions on Atoms and Nuclei
EASY
JEE Main
IMPORTANT
The energy level diagram for a hydrogen-like atom is shown in the figure. The radius of its first Bohr orbit is

EASY
JEE Main
IMPORTANT
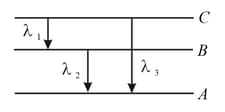
Energy levels , , of a certain atom corresponding to increasing values of energy, i.e., . If , and are the wavelengths of radiations corresponding to the transitions to , to and to respectively, which of the following statements is correct
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
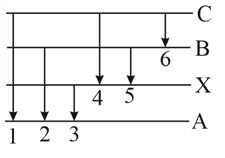
In the figure, six lines of emission spectrum are shown. Which of them will be absent in the absorption spectrum?
EASY
JEE Main
IMPORTANT
