MEDIUM
11th CBSE
IMPORTANT
Earn 100
The ratio of specific charge of an electron to that of a hydrogen ion is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
As electron moves away from the nucleus, its
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
Three energy levels and the wavelengths of the lines produced by transitions are shown in the figure below:
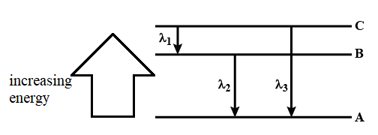
Which one of the following relationships is correct?
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
