EASY
Earn 100
The ratio of the slopes of graphs of adiabatic and isothermal is
(a)
(b)
(c)
(d)γ+1
66.67% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
Two moles of an ideal monoatomic gas occupies a volume at . The gas expands adiabatically to a volume . Calculate the final temperature of the gas and change in its internal energy.
EASY
EASY
EASY
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 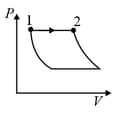 |
| (II) | (ii) Isochoric | (Q) 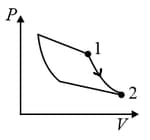 |
| (III) | (iii) Isobaric | (R) 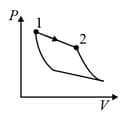 |
| (IV) | (iv) Adiabatic | (S) 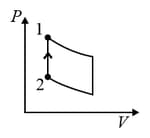 |
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 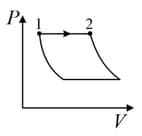 |
| (II) | (ii) Isochoric | (Q) 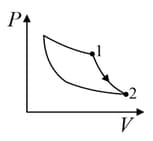 |
| (III) | (iii) Isobaric | (R) 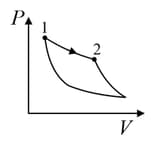 |
| (IV) | (iv) Adiabatic | (S) 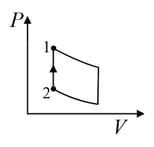 |
EASY
HARD
EASY
HARD
HARD
EASY
HARD
HARD
(Given is gas constant)
MEDIUM
HARD

