The reaction of calcium cyanamide with water yields
Important Questions on The s-Block Elements
Given below are two statements, one is labelled as
Assertion A and the other is labelled as Reason R.
Assertion A : Beryllium has less negative value of reduction potential compared to the other alkaline earth metals
Reason R : Beryllium has large hydration energy due to small size of but relatively large value of atomisation enthalpy.
In the light of the above statements, choose the most appropriate answer from the options given below.
Reaction of with gives :
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from options given below
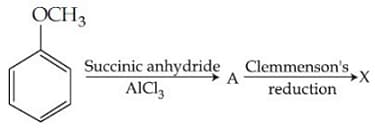
X is :
Given below are two statements :
Statement I : Lithium and Magnesium do not form superoxide
Statement II : The ionic radius of is larger than ionic radius of In the light of the above statements, choose the most appropriate answer from the questions given below :
In the light of the above statements, choose the most appropriate answer from the questions given below :
Among the statement (I – IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than AI.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds
