EASY
Earn 100
The reaction of propene with HOCl proceeds via the addition of –
(a)H+ in the first step
(b)Cl+ in the first step
(c)OH- in the first step
(d)Cl+ and OH- single step
50% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
In the given reaction, the product is
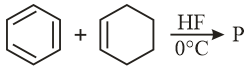
MEDIUM
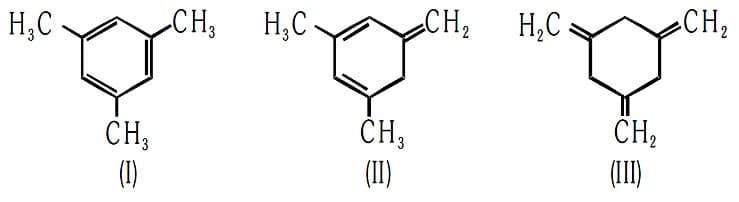
The enthalpy of hydrogenation of these compounds will be in the order as:
EASY
MEDIUM
MEDIUM
The major product of the following reaction is:
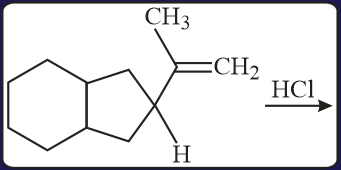
MEDIUM
HARD
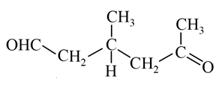
is obtainable from ozonolysis of which of the following cyclic compounds?
HARD
EASY
HARD
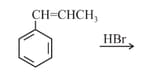
What is the major product expected from the following reaction?
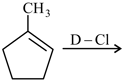
Where D is an isotope of hydrogen.
MEDIUM
HARD
The major product of the following reaction is:
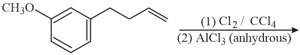
MEDIUM
MEDIUM

HARD
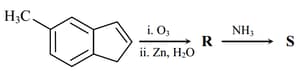
MEDIUM
The correct statement(s) for the following addition reactions is(are)
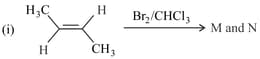
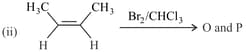
HARD
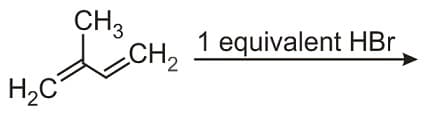
HARD
HARD
The major product in the following conversion is
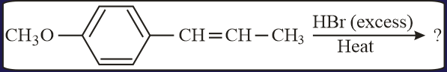
HARD

