The reactivity series lists metals in order of reactivity. To find out which is more reactive, zinc or tin, this experiment could be carried out.
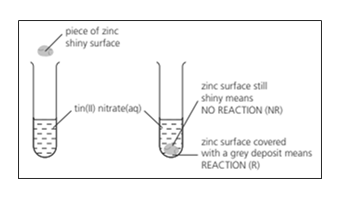
The experiment could be carried out with other metals and the results recorded in a table. Then the order of reactivity can be deduced. The order was found to be:
aqueous solution
Tin
Manganese
Silver
Zinc
tin nitrate
-
manganese nitrate
silver nitrate
zinc nitrate
Copy and complete this table of results from which the order was determined.


Important Questions on Cambridge IGCSE Exam Questions from Paper 3
The reactivity series lists metals in order of reactivity. To find out which is more reactive, zinc or tin, this experiment could be carried out.
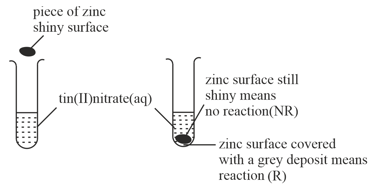
Write the ionic equation for the reaction between tin atoms and silver ions.
Potassium and calcium are very reactive metals at the top of the series. Because their ions have different charges, and , their compounds behave differently when heated. Their hydroxides are heated. If the compound decomposes, complete the word equation. If it does not decompose, write 'no reaction'.
_____.
_____.
Complete the equations for the decomposition of their nitrates:
_____ _____
_____ _____ _____
The fractional distillation of crude oil usually produces large quantities of the heavier fractions. The market demand is for the lighter fractions and for the more reactive alkenes. The heavier fractions are cracked to form smaller alkanes and alkenes as in the following example:
Write a different equation for the cracking of octane:
The cracking of octane can produce isomers with the molecular formula . Draw the structural formulae of two of these isomers.
