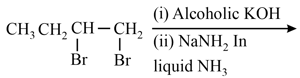MEDIUM
Earn 100
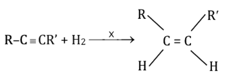
The reduction of an alkyne to alkene using lithium metal in liquid ammonia as solvent results into
(a) addition of hydrogen atoms
(b) addition of hydrogen atoms
(c)Both and additions of hydrogen atoms. The relative amounts of the two depends on temperature
(d)Both and additions of hydrogen atoms. The relative amounts depend on the nature of alkyne
50% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
EASY
HARD
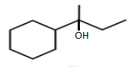 ?
?EASY
MEDIUM
Consider the following reactions:
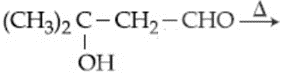
Which of the reaction(s) will not produce Saytzeff product?
EASY
MEDIUM
EASY
HARD
HARD
The major product in the following sequence of reaction is :
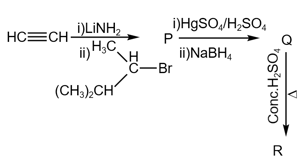
EASY
MEDIUM
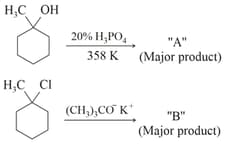
The product "" and "" formed in above reactions are
HARD
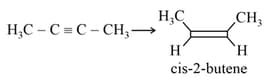
EASY
HARD