EASY
Earn 100
The relative number and the kind of atoms in a given compound are constant. This postulate of Dalton's atomic theory can explain the law of
.
50% studentsanswered this correctly
Important Questions on Atoms and Molecules
MEDIUM
The reaction of the burning of carbon in oxygen is represented by equation
When 9.0 g of solid carbon is burnt in 16.0 g of oxygen gas the mass of carbon dioxide gas formed would be:
(Note: Atomic mass of C-12,o u, O=16.0 u)
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
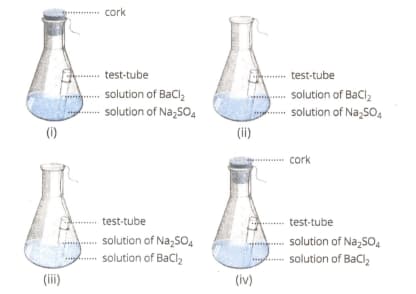
Select from the following figure(s) that correctly represent(s) the experimental set-up for the verification of conservation of mass in a chemical reaction.