EASY
JEE Main
IMPORTANT
Earn 100
The relative strength of the interionic/ intermolecular forces in a decreasing order is:
(a)dipole-dipole ion-dipole ion-ion
(b)ion-dipole ion-ion dipole-dipole
(c)ion-dipole dipole-dipole ion-ion
(d)ion-ion ion-dipole dipole-dipole
45.45% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main
IMPORTANT
For gaseous state, if most probable speed is denoted by , average speed by and root mean square speed by , then for many molecules, what is the ratios of these speeds?
HARD
JEE Main
IMPORTANT
If Z is the compressibility factor, then Van der Waal's equation at low pressure can be written as:
EASY
JEE Main
IMPORTANT
A spherical balloon of radius containing helium gas has a pressure of bar. At the same temperature, the pressure, of a spherical balloon of radius containing the same amount of gas will be bar.
EASY
JEE Main
IMPORTANT
The intermolecular interaction that is dependent on the inverse cube of distance between the molecules is:
HARD
JEE Main
IMPORTANT
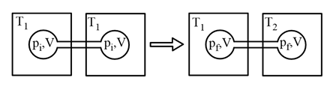
Two closed bulbs of equal volume containing an ideal gas initially at pressure and temperature are connected through a narrow tube of negligible volume, as shown in the figure below. The temperature of one of the bulbs is then raised to The final pressure is:
EASY
JEE Main
IMPORTANT
Which one of the following is the correct vs plot at constant temperature for an ideal gas ? ( and stand for pressure and volume of the gas respectively)
EASY
JEE Main
IMPORTANT
A mixture of one mole each of , and each are enclosed in a cylinder of volume at temperature T. If the partial pressure of is the total pressure of the gases in the cylinder is:
EASY
JEE Main
IMPORTANT
Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
