MEDIUM
JEE Main
IMPORTANT
Earn 100
The resonating structures of the cyanate ion are 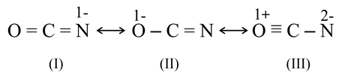 . The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
. The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Redox Reactions
MEDIUM
JEE Main
IMPORTANT
is oxidised by in the presence of an acid : . What are the whole number values of in that order?
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
The number of moles of that will be needed to react completely with one mole of ferrous oxalate in an acidic solution is:
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
of ammonia at and atmospheric pressure is neutralised by of solution. Find the normality of the acid.
Give the answer after rounding-off to two places of decimal.
