MEDIUM
NEET
IMPORTANT
Earn 100
The speed of a molecule of oxygen at is half that of a molecule of hydrogen at
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Kinetic Theory
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
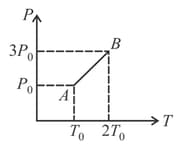
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point is Density at point will be
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
