MEDIUM
NEET
IMPORTANT
Earn 100
The solubility curve of as a function of temperature is given below:
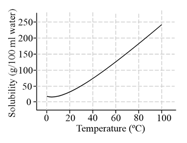
The amount of that will crystallize when a saturated solution of in of water is cooled from to , is:

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
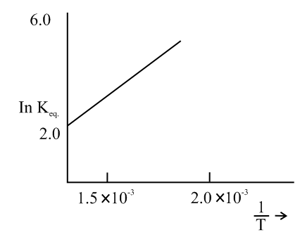
The graph given below relates for a reaction. The reaction must be
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
