EASY
NEET
IMPORTANT
Earn 100
The solubility curve of in water is shown below:
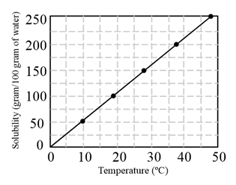
The amount of that dissolves in of water at is closest to:

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
For the reaction:
Which one is correct representation?
EASY
NEET
IMPORTANT
For a reaction , the value of does not depend upon:
Initial concentration of the reactants
Pressure
Temperature
catalyst
MEDIUM
NEET
IMPORTANT
is atmosphere. The value of in terms of would be:
