EASY
Earn 100
The solubility of haloalkanes is greater in water.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
MEDIUM
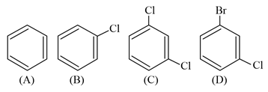
The correct order of melting point of dichlorobenzenes is
MEDIUM
Why solubility of Haloalkanes in water is very low?
MEDIUM
Identify the correct order for the given property for following compounds
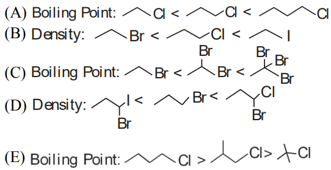
Choose the correct answer from the option given below :-
MEDIUM
The major product of the following reaction is:
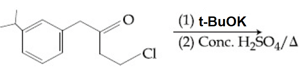
EASY
Arrange the following compounds in the decreasing order of boiling points:
EASY
For the compounds CH3Cl, CH3Br, CH3I and CH3F, the correct order of increasing C-halogen bond length is :
MEDIUM
Which among the following will have highest density?
MEDIUM
Which of the following will most readily give the dehydrohalogenation product?
EASY
Give reasons:
Haloalkanes easily dissolve in organic solvents.
EASY
Give reasons:
C — Cl bond length in chlorobenzene is shorter than C — Cl bond length in chloromethane.
MEDIUM
The compound on reaction with chlorine in presence of light gives ; which when reacted with in the solvent -dimethylformamide, gives -Nitrobutane. The compound is
HARD
Explain why alkyl halides, though polar, are immiscible with water?
EASY
The correct pair(s) of the ambident nucleophiles is (are):
(A)
(B)
(C)
(D)
EASY
Consider the reaction
This reaction will be the fastest in
EASY
Give reason:
n-Butyl bromide has higher boiling point than t-Butyl bromide.
EASY
The correct decreasing order of densities of the following compounds is :
HARD
The increasing order of the boiling points of the major products and of the following reactions will be :
(a)
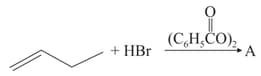
(b)
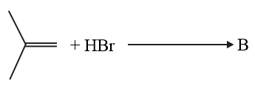
(c)
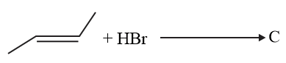
MEDIUM
Which of the following compound will have highest melting point?
MEDIUM
Which one of the following possess highest melting point?
EASY
Which of the following will have maximum dipole moment and maximum boiling point respectively?

