MEDIUM
Earn 100
The solubility product of is . The solubility of in buffer solution of pH = 8 is
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Ionic Equilibrium
HARD
An aqueous solution contains an unknown concentration of . When solution of is added, just begins to precipitate. The final volume is . The solubility product of is . What is the original concentration of ?
EASY
If of is , the molar solubility of in is:
HARD
Zirconium phosphate dissociates into three zirconium cations of charge and four phosphate anions of charge . If molar solubility of zirconium phosphate is denoted by and its solubility product by then which of the following relationship between and is correct ?
MEDIUM
pH of a saturated solution of is . The solubility product of is:
HARD
The solubility of a salt of weak acid . The value of is ____(Nearest integer). (Given that the value of solubility product of , and the value of ionisation constant of )
MEDIUM
What is the molar solubility of in solution? Given that, solubility product of :
MEDIUM
When is added to a aqueous solution of the concentration of . If the same amount of is added to a aqueous solution of the concentration of is
MEDIUM
The solubility product of at is The concentration of hydroxide ions in a saturated solution of will be
EASY
The solubility of with solubility product in solution would be
HARD
Consider the electrochemical reaction between and electrodes in of aqueous solution. Solubility product of is and . At current, calculate the time required to start observing the precipitation in the galvanic cell
MEDIUM
The molar solubility of is in water. The expected solubility of in a buffer solution of is:
MEDIUM
is precipitated when is added to a solution of . If the final concentration of is M, the concentration of in the solution is
[Solubility product for ]
MEDIUM
The for the following dissociation is
Which of the following choices is correct for a mixture of and
MEDIUM
If solubility product of is denoted by and its molar solubility is denoted by , then which of the following relation between and is correct?
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
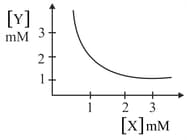
EASY
The for bismuth sulphide is . The solubility of in at is
EASY
Solubility of is least in
MEDIUM
Concentration of the ions in a saturated solution of is . Solubility product of is:
HARD
The of and are respectively, . Which one of the following salts will precipitate last if solution is added to the solution containing equal moles of NaCl, NaI, NaBr and ?
MEDIUM
MY and , two nearly insoluble salts, have the same values of at room temperature. Which statement would be true in regard to MY and ?

