HARD
12th ICSE
IMPORTANT
Earn 100
The specific conductivity of a solution containing g of anhydrous ( molecular weight) in of a solution is found to be . Calculate the molar and equivalent conductivity of the solution.

Important Questions on Electrochemistry
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
Calculate the mass of silver deposited at cathode when a current of amperes is passed through a solution of for minutes. (atomic mass: ).
MEDIUM
12th ICSE
IMPORTANT
Calculate the emf and for the cell reaction at ·
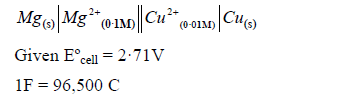
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
The resistance of a conductivity cell containing solution at is . What is the cell constant and molar conductivity of solution, if the conductivity of this solution is at ?
EASY
12th ICSE
IMPORTANT
