The specific heat capacity of a substance is the amount of heat required to raise the temperature of of the substance by . The specific heat capacities of a few substances are also given here. Using this information, answer the question.
Substance
Specific heat ()
Water
Vegetable oil
Kerosene
Alcohol
Aluminium
Copper
Iron
Lead
For storing thermal energy in solar heating systems, we would need a material that retains heat the longest. Which of these would be most suited?

Important Questions on Heat
The specific heat capacity of a substance is the amount of heat required to raise the temperature of of the substance by . The specific heat capacities of a few substances are also given here. Using this information, answer the question.
| Substance | Specific heat () |
| Water | |
| Vegetable oil | |
| Kerosene | |
| Alcohol | |
| Aluminium | |
| Copper | |
| Iron | |
| Lead |
If equal masses each of water, vegetable oil, aluminium and copper are heated uniformly for five minutes, which one of them will record the maximum rise in temperature?
A Maximum-Minimum thermometer has two stems which record the highest and lowest temperatures during a period of time. A small steel index in each tube indicates maximum and minimum temperature.
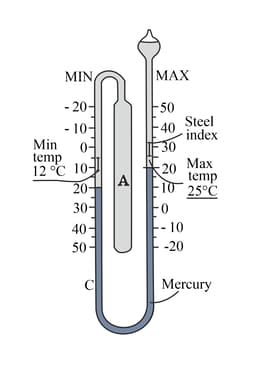
What are the maximum, minimum and current temperatures shown in the following thermometer.
A device used for measuring the temperature of different objects is called a thermometer. The thermometer consists of a very fine glass tube which consists of a thin glass bulb at one end. The bulb is filled with thermometric liquid. After removing air, the other end of tube is sealed. The tube is protected by a thick glass tube called stem. On the stem the markings are given.
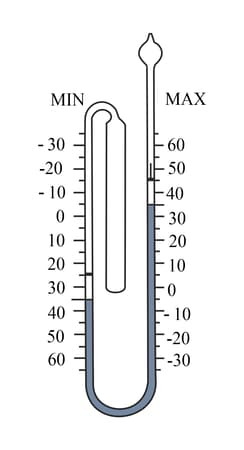
Which of these readings cannot be taken with the thermometer shown?
Two identical vessels are taken and painted white and black respectively. Then the same quantity of water is poured into each one of them. Both the vessels are left in the sun, and the temperature is noted regularly. The water temperature in the vessel painted white is recorded every five minutes as shown in the table below.
| Water temperature in white vessel | |
|---|---|
| After minutes | |
| After minutes | |
| After minutes | |
| After minutes | |
What is the temperature in the vessel likely to be minutes after it was left in the sun?
Rani has two bowls in front of her. Bowl P contains grams of ice, while bowl Q contains three kilograms of the same material. Both are heated and start melting. Which of the following will be true about the temperature at which the ice just starts melting, and the temperature at which the wax melts completely.
| Temperature at which ice starts melting | Temperature at which ice just melts completely | |
| A | Same for both | Lower for P |
| B | Lower for P | Lower for P |
| C | Same for both | Same for both |
| D | Lower for P | Same for both |
