HARD
Physics
IMPORTANT
Earn 100
The specific heat of many solids at low temperatures varies with absolute temperature according to the graph as shown. Then heat energy required to raise the temperature of a unit mass of such a solid from to
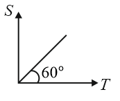

(a)
(b)
(c)
(d)
37.5% studentsanswered this correctly

Important Questions on Thermal Properties of Matter
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
