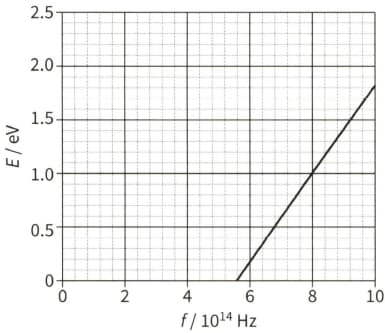
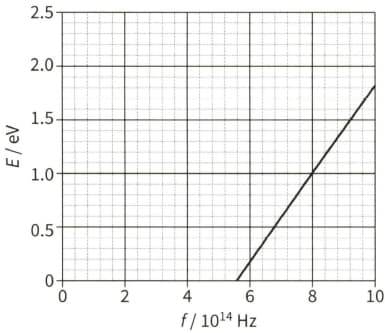
The spectrum of sunlight has dark lines. These dark lines are due to the absorption of certain wavelengths by the cooler gases in the atmosphere of the Sun.
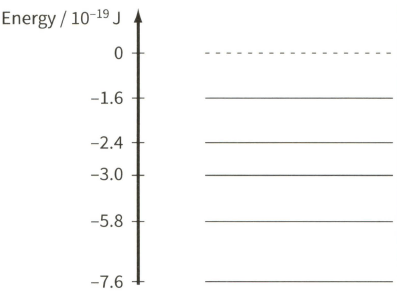
The diagram shows some of the energy levels of an isolated atom of helium.
All the light absorbed by the atoms in the Sun's atmosphere is re-emitted. Suggest why a dark spectral line of wavelength of $590 \mathrm{~nm}$ is still observed from the Earth.


Important Questions on Quantum Physics
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
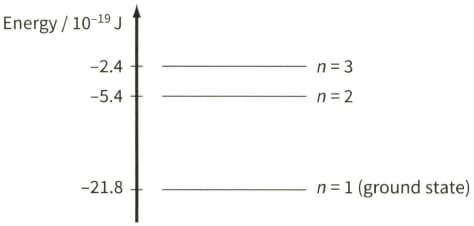
Explain what happens to an electron in the ground state when it absorbs the energy from a photon energy $21.8 \times 10^{-19} \mathrm{~J}$.
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
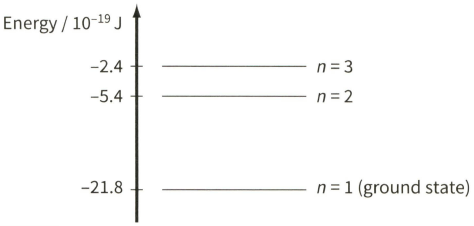
Explain why a photon is emitted when an electron makes a transition between energy levels $n=3$ and $n=2 .$
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
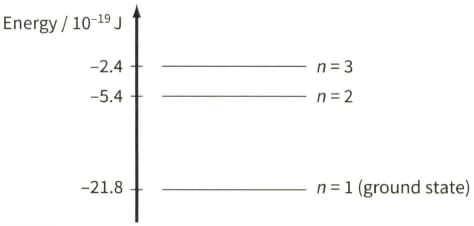
Calculate the wavelength of electromagnetic radiation emitted when an electron makes a jump between energy levels $n=3$ and $n=2$.
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
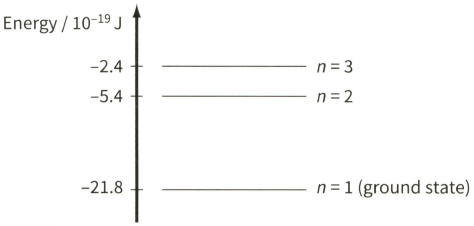
In the diagram, each energy level is labelled with its 'principal quantum number' $n$. Use the energy level diagram to show that the energy $E$ of an energy level is inversely proportional to $n^{2} .$
Explain how the photoelectric effect gives evidence for the wave-particle duality of electromagnetic radiation.