MEDIUM
Earn 100
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at . The equilibrium constant of the reaction is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Redox Reactions and Electrochemistry
EASY
MEDIUM
at is approximately:
MEDIUM
HARD
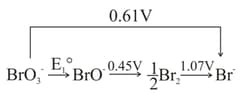
The value of is
EASY
MEDIUM
MEDIUM
MEDIUM
The standard emf of the cell and equilibrium constant of the following reaction of
EASY
EASY
MEDIUM
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
EASY
MEDIUM
is
EASY
MEDIUM
The standard e.m.f. of the cell, in which the cell reaction is is at and at . The enthalpy change of the reaction at is
MEDIUM
MEDIUM
MEDIUM
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
EASY
Given that:
HARD
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and

